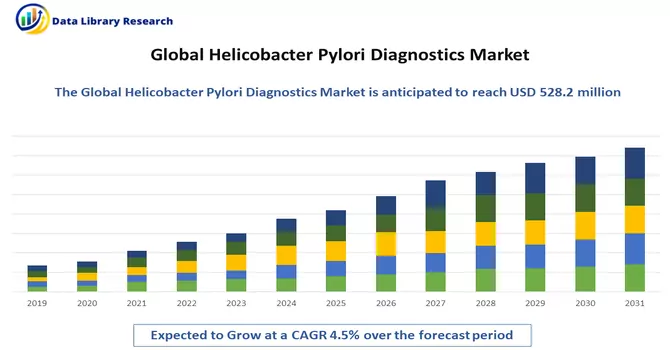
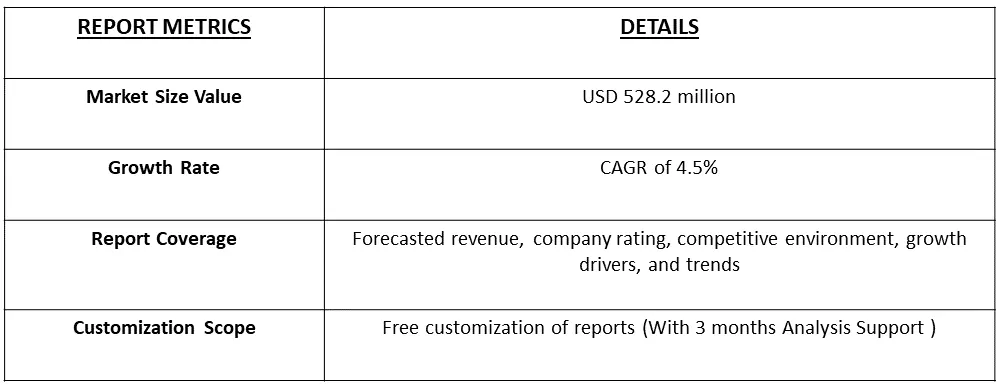
The global market size for helicobacter pylori diagnostics reached an estimated USD 528.2 million, with projections indicating a steady compound annual growth rate (CAGR) of 4.5% from 2023 to 2030.
Get Complete Analysis Of The Report - Download Free Sample PDF
Helicobacter pylori diagnostics refer to the various methods and procedures employed to identify and detect the presence of Helicobacter pylori (H. pylori) bacteria in the human body. H. pylori is a type of bacteria that can infect the stomach and is a common cause of gastritis and peptic ulcers. Diagnosing H. pylori infection is crucial for managing and treating associated gastrointestinal conditions. Diagnostic methods for H. pylori include non-invasive techniques such as blood tests, breath tests, and stool antigen tests, as well as invasive methods like endoscopy with biopsy. These diagnostic approaches help healthcare professionals determine whether an individual is infected with H. pylori, enabling timely intervention and appropriate treatment to alleviate symptoms and prevent complications.
The key drivers behind this growth include the growing demand for personalized antibiotic therapy, an upswing in infectious diseases, enhanced cost-effectiveness in helicobacter pylori infection diagnosis, and increased public awareness about infections. These factors collectively contribute to the expanding landscape of the helicobacter pylori diagnostics market.
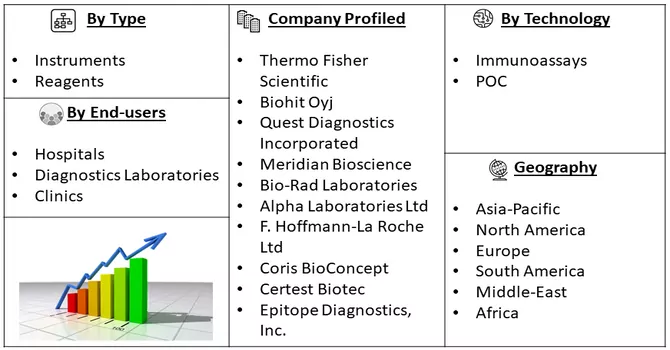
Market Segmentation: Helicobacter Pylori Diagnostics Market Size, Share & Trends Analysis Report By Type (Instruments, and Reagents), By Technology (Immunoassays, and POC), End-users (Hospitals, Diagnostics Laboratories and Clinics) and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The report offers market size and forecast values in USD million for the above segments.
For Detailed Market Segmentation - Download Free Sample PDF
There is a growing trend toward personalized antibiotic therapy based on the specific strain of Helicobacter pylori present in an individual. Tailoring treatment to the unique characteristics of the infection enhances efficacy and reduces the risk of antibiotic resistance. The overall surge in infectious diseases, including gastrointestinal infections, is contributing to the demand for Helicobacter pylori diagnostics. As these infections become more prevalent, the need for accurate and timely diagnosis is on the rise.
Moreover, ongoing technological advancements in diagnostic tools and techniques, such as molecular diagnostics and rapid testing methods, are enhancing the accuracy and efficiency of Helicobacter pylori diagnostics. This trend is influencing the market by offering innovative solutions to healthcare providers.
Market Drivers :
Increasing Prevalence of Helicobacter pylori Infections
The rising incidence of Helicobacter pylori infections globally is a significant driver for the diagnostics market. As the prevalence of these infections continues to grow, the demand for accurate and timely diagnostic tools increases. An article titled, ‘Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis” published in Auguust 2017, reported that among 14,006 reports screened, the researchers has identified 263 full-text articles on the prevalence of H pylori infection; 184 were included in the final analysis, comprising data from 62 countries. Africa had the highest pooled prevalence of H pylori infection (70.1%; 95% CI, 62.6-77.7), whereas Oceania had the lowest prevalence (24.4%; 95% CI, 18.5-30.4) Thus, these statistic shows that the market is expected to witness significant growth over the forecast period.
Growing Awareness and Understanding of Gastrointestinal Health
The increasing awareness among individuals and healthcare professionals about the importance of gastrointestinal health and the role of Helicobacter pylori in related disorders is driving the demand for diagnostic testing. The role of Helicobacter pylori in gastrointestinal disorders has prompted individuals to prioritize preventive measures and early intervention. With increased awareness, individuals are more likely to seek diagnostic testing to detect H. pylori infections at an early stage. Moreover, as awareness grows, so does the recognition of the prevalence of gastrointestinal disorders associated with Helicobacter pylori. This understanding prompts healthcare professionals to recommend diagnostic testing as a routine or targeted measure for individuals presenting with gastrointestinal symptoms. Thus, these factors are expected to dive the growth of the studied market over the forecast period.
Market Restraints
Limited Accessibility in Developing Regions and Challenges in Accuracy and Specificity
Limited access to advanced diagnostic technologies and healthcare infrastructure in developing regions poses a significant restraint. This can hinder the widespread adoption of Helicobacter pylori diagnostics in areas with fewer resources. Moreover, despite advancements, some diagnostic methods may face challenges in terms of accuracy and specificity. False-positive or false-negative results can undermine confidence in the diagnostic process, impacting its effectiveness. Thus, these factors are expected to slow the growth of the studied market over the forecast period.
The pandemic has led to a significant disruption in routine healthcare services, with a focus on managing and containing the spread of the virus. This diversion of attention and resources has, in some cases, affected the prioritization of diagnostic testing for conditions such as Helicobacter pylori infections. Also, the pandemic has prompted a shift in healthcare priorities towards infectious disease management, vaccination efforts, and pandemic response. This shift may have temporarily diverted attention and resources away from non-communicable diseases, including gastrointestinal infections like Helicobacter pylori. Thus, the COVID-19 pandemic has presented challenges and opportunities for the global Helicobacter pylori diagnostics market. The extent and duration of these impacts will depend on the ongoing dynamics of the pandemic, the resilience of healthcare systems, and the adaptation of diagnostic providers to the evolving landscape.
Segmental Analysis
Instrument Segment is Expected To Witness Significant growth Over the Forecast Period
In the field of Helicobacter pylori diagnostics, various instruments play a crucial role in detecting the presence of H. pylori bacteria in clinical samples. These instruments contribute to the accuracy, efficiency, and speed of diagnostic processes, impacting the overall Helicobacter pylori diagnostics market. Endoscopy is a common method for diagnosing Helicobacter pylori infections. Instruments such as gastroscopes are used to visualize the stomach lining, and during the procedure, biopsies may be taken for further analysis.
Also, polymerase chain reaction (PCR) is a molecular diagnostic technique used to amplify and analyze DNA. PCR machines are integral to molecular diagnostic methods for Helicobacter pylori, enabling the detection of specific genetic material associated with the bacterium. Thus, due to applications of various instruments in detection bacteria, the segment is expected to witness significant growth over the forecast period.
Immunoassays Segment is Expected to Witness Significant growth over the Forecast Period
Immunoassays are a critical component of the global Helicobacter pylori diagnostics market, offering a highly sensitive and specific method for detecting antibodies or antigens associated with H. pylori infections. ELISA is a widely used immunoassay technique in Helicobacter pylori diagnostics. It involves the detection of antibodies or antigens through an enzymatic reaction. ELISA kits are designed to provide a quantitative or qualitative assessment of the presence of H. pylori markers in patient samples. Thus, the widespread adoption of immunoassays in Helicobacter pylori diagnostics reflects their versatility, speed, and applicability across different healthcare settings. As technology continues to advance, immunoassay techniques are likely to play a crucial role in the development of innovative diagnostic solutions for H. pylori infections and thus contributing to the studied segment’s growth.
Diagnostic Labs Segment is Expected to Witness Significant growth over the Forecast Period
Diagnostics laboratories receive various types of clinical samples, including blood, stool, and tissue specimens, collected for the detection of Helicobacter pylori. These samples undergo meticulous processing and analysis to identify the presence of H. pylori antigens, antibodies, or genetic material. Some diagnostics laboratories are involved in research and development activities aimed at advancing diagnostic technologies for H. pylori infections. This includes the exploration of novel biomarkers, improvement of testing methodologies, and the integration of innovative technologies. Thus, diagnostics laboratories serve as critical hubs in the detection and diagnosis of Helicobacter pylori infections. Their role extends beyond processing samples to encompass quality control, collaboration with healthcare professionals, and ongoing contributions to research and development in the field of H. pylori diagnostics, thereby driving the growth of the segment, over the forecast period.
North America Region is Expected to Witness Significant growth over the Forecast Period
North America, comprising the United States and Canada, represents a substantial share of the global Helicobacter pylori diagnostics market. The region's advanced healthcare infrastructure and high awareness levels contribute to its significant market size. The innovations and trends originating in North America significantly influence the global Helicobacter pylori diagnostics market. The region's research and development activities contribute to shaping the direction of diagnostic technologies worldwide.
Moreover, companies based in North America employ market expansion strategies that go beyond regional borders. This includes entering new markets, forming international alliances, and tailoring diagnostic solutions to meet the diverse needs of global healthcare systems. Thus, these factors are expected to drive the growth of the studied market in the region over the forecast period.
Get Complete Analysis Of The Report - Download Free Sample PDF
Enterprises are implementing diverse strategies, including the development of new products, engaging in mergers and acquisitions, forming strategic partnerships, and expanding their presence in different regions. These approaches are strategically employed to enhance competitiveness and strengthen their position in the market. Moreover, Companies are actively focusing on creating and introducing innovative products to meet evolving market demands. The emphasis on research and development allows organizations to offer cutting-edge solutions, staying ahead of the competition and catering to changing customer needs. Some prominent players in the global Helicobacter pylori diagnostics market include:
Recent Development:
1) In May 2022, Biomerica, a biomedical technology company, disclosed that it had received the CE Mark for hp+detect, a diagnostic test designed for the detection of H. pylori. Following the product's registration in the respective countries, the company anticipates selling this diagnostic test in the European Union and other nations.
2) In April 2021, Otsuka Pharmaceutical Co., Ltd. introduced QuickNavi, a rapid H. pylori detection kit capable of delivering test results within eight minutes. Conversely, in October 2018, DiaSorin S.p.A. entered into a collaborative partnership with Meridian Bioscience, Inc. to market DiaSorin's FDA-approved stool antigen test designed for the detection of H. pylori.
Q1. How big is the Helicobacter Pylori Diagnostics Market ?
The global market size for helicobacter pylori diagnostics reached an estimated USD 528.2 million, with projections indicating a steady CAGRof 4.5%.
Q2. At what CAGR is the Helicobacter Pylori Diagnostics Market projected to grow within the forecast period?
Helicobacter Pylori Diagnostics Market is expected to grow at a compound annual growth rate (CAGR) of 4.5% over the forecast period.
Q3. What are the Growth Drivers of the Helicobacter Pylori Diagnostics Market?
Increasing Prevalence of Helicobacter pylori Infections and Growing Awareness and Understanding of Gastrointestinal Health are the Growth Drivers of the Helicobacter Pylori Diagnostics Market
Q4. What segments are covered in the Helicobacter Pylori Diagnostics Market Report?
By Type, By Application, End-User and Geography these segments are covered in the Helicobacter Pylori Diagnostics Market Report.
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
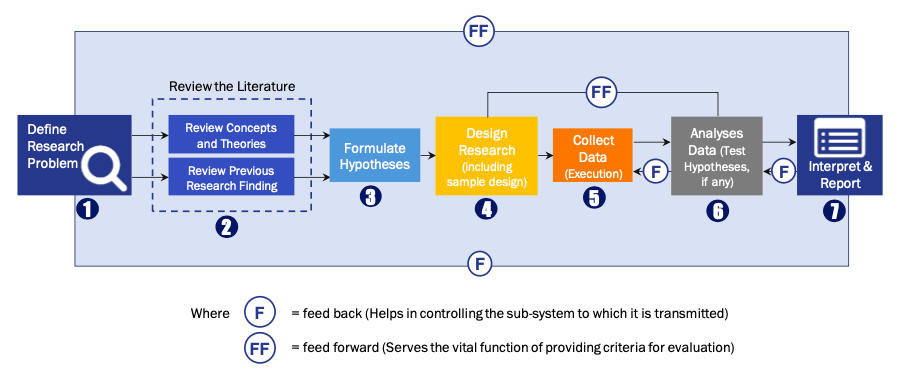
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model