Biosimilars Market Overview A biosimilar is a biological product that is very similar to a reference biologic and for which there are no clinically meaningful differences in terms of safety, purity, and potency. The global Biosimilars market is expected to grow at a sound pace in the times to follow. The growing aging population and changing societal behavior are the vital factors contributing to the rising incidence of chronic diseases. Major chronic diseases include diabetes, hypertension, respiratory diseases, oral diseases, obesity, arthritis, and cancer. Therefore, increasing chronic diseases globally is one of the key factors to boosts the growth of the global Biosimilars market.
Additionally, biosimilars are the copy versions of biologics, which have reached the end of patent protection. The patent expiries of major biologics are anticipated to contribute more to the growth of the Biosimilars market in the coming years. Other prime factors which are fuelling the growth of the market are significant product pipeline, the focus of market players on expanding their presence in emerging markets, and cost savings offered by biosimilars.
Furthermore, the approvals of biosimilars have been increasing worldwide. This is because of changing rules and increasing pressure on the healthcare system to facilitate treatment to all patients. In addition to this, biosimilars are being progressively adopted by physicians, authorities, and patients, due to much-needed improvements to access therapeutically viable treatments for several diseases. All these aforementioned aspects are projected to contribute remarkably to the growth of the Biosimilars market in the next few years.
Covid-19 Impact on Biosimilars Market In addition, the current Biosimilars Market study offers a detailed analysis of the current COVID-19 pandemic impact on the market growth and its influence on the future growth of the Biosimilars Market. The recently published report demonstrates the elevation in the demand for the healthcare sector. The healthcare manufacturers have experienced long term as well as short term effect which includes supply shortages, panic buying, and stocking, regulation changes as short-term whereas approval delays and possible trend variations in consumption could be perceived as long-term impacts of COVID-19 on the health and pharmaceutical market.
The increasing need for a cure has pushed vaccine research and manufacturers to the limit. In addition to this, panic conditions have already spurred the demand for many healthcare products and services which are discussed in detail in this report. Moreover, the impact of COVID-19 on overall market revenue for the base year 2020 and its projection up to 2027 is provided in detail in this report.
Biosimilars Market Segment OverviewBased on the product, the Monoclonal Antibody segment accounted for the highest share of the Biosimilars market in 2019. This is attributed mainly to the increasing incidence of cancer and the growing number of product launches/approvals. Moreover, according to Indication, the Oncology segment dominated the Biosimilars market. It is predictable to maintain its dominance throughout the forecast period. This is due to the increase in the prevalence of cancer and a significant pipeline of biosimilars focused on the treatment of cancers.
Biosimilars Market, By Product
- Recombinant Glycosylated Proteins
- Monoclonal Antibodies
- Erythropoietin
- Others
- Recombinant Non-glycosylated Proteins
- Insulin
- Granulocyte Colony Stimulating Factor
- Recombinant Human Growth Factor
- Interferons
- Recombinant Peptides
Biosimilars Market, By Indication
· Chronic Diseases
· Oncology
· Autoimmune Diseases
· Infectious Diseases
· Blood Disorders
· Growth Hormone Deficiency
· Others
Biosimilars Market Regional OverviewGeographically, Europe has constituted the largest market for biosimilars. This region accounts for over 70% of the global biosimilar spending, as it is the most developed biosimilar market. Presently, Europe has over 60 approved biosimilars and contributing significantly to the growth of the biosimilars market in this region. Similarly, Asia Pacific is expected to emerge as a lucrative region for the Biosimilars market. The healthy growth of the market in this region can be attributed to increasing manufacturing of biosimilars in India and Korea. In addition, there are above 300 biosimilars under development in Asia, this factor helps to boost the position of Asia Pacific as the center for global biosimilars manufacturing and adoption.
Biosimilars Market, By Geography
· North America (US & Canada)
· Europe (UK, Germany, France, Italy, Spain, Russia & Rest of Europe)
· Asia-Pacific (Japan, China, India, Australia, & South Korea, & Rest of Asia-Pacific)
· LAMEA (Brazil, Saudi Arabia, UAE & Rest of LAMEA)
Biosimilars Market Competitor overviewSome key developments and strategies adopted by manufacturers in Biosimilars are highlighted below.
· In July 2021, FDA approved Mylan Pharmaceuticals’ Semglee (insulin glargine-yfgn), the first interchangeable biosimilar insulin product in the United States for treating diabetes. The product is indicated for improving glycemic control in adults and pediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. Semglee is both biosimilar to and interchangeable with its reference product, Lantus (insulin glargine), a long-acting insulin analog.
Biosimilars Market, Key Players· Pfizer, Inc.
· Intas Pharmaceuticals Ltd.
· Biocon
· Dr. Reddy’s Laboratories Ltd.
· Teva Pharmaceutical Industries Ltd.
· Sandoz International GmbH (A Novartis Division)
· Celltrion Inc.
· Amgen, Inc.
· STADA Arzneimittel AG
· Apotex Inc. (Apobiologix)
Biosimilars Market Study Global Market Analysis, Insights and Forecast, 2020-2027
1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
2. Executive Summary
3. Market Dynamics
- 3.1. Market Drivers
- 3.2. Market Restraints
- 3.3. Market Opportunities
4. Key Insights
- 4.1. Key Emerging Trends – For Major Countries
- 4.2. Latest Technological Advancement
- 4.3. Regulatory Landscape
- 4.4. Industry SWOT Analysis
- 4.5. Porters Five Forces Analysis
5. Global Biosimilars Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 5.1. Key Findings / Summary
- 5.2. Market Analysis, Insights and Forecast – By Product
- 5.2.1. Recombinant Glycosylated Proteins
- 5.2.1.1. Monoclonal Antibodies
- 5.2.1.2. Erythropoietin
- 5.2.1.3. Others
- 5.2.2. Recombinant Non-glycosylated Proteins
- 5.2.2.1. Insulin
- 5.2.2.2. Granulocyte Colony Stimulating Factor
- 5.2.2.3. Recombinant Human Growth Factor
- 5.2.2.4. Interferons
- 5.2.3. Recombinant Peptides
- 5.3. Market Analysis, Insights and Forecast – By Indication
- 5.3.1. Chronic Diseases
- 5.3.2. Oncology
- 5.3.3. Autoimmune Diseases
- 5.3.4. Infectious Diseases
- 5.3.5. Blood Disorders
- 5.3.6. Growth Hormone Deficiency
- 5.3.7. Others
- 5.4. Market Analysis, Insights and Forecast – By Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Latin America, Middle East and Africa
6. North America Biosimilars Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 6.1. Key Findings / Summary
- 6.2. Market Analysis, Insights and Forecast – By Product
- 6.2.1. Recombinant Glycosylated Proteins
- 6.2.1.1. Monoclonal Antibodies
- 6.2.1.2. Erythropoietin
- 6.2.1.3. Others
- 6.2.2. Recombinant Non-glycosylated Proteins
- 6.2.2.1. Insulin
- 6.2.2.2. Granulocyte Colony Stimulating Factor
- 6.2.2.3. Recombinant Human Growth Factor
- 6.2.2.4. Interferons
- 6.2.3. Recombinant Peptides
- 6.3. Market Analysis, Insights and Forecast – By Indication
- 6.3.1. Chronic Diseases
- 6.3.2. Oncology
- 6.3.3. Autoimmune Diseases
- 6.3.4. Infectious Diseases
- 6.3.5. Blood Disorders
- 6.3.6. Growth Hormone Deficiency
- 6.3.7. Others
- 6.4. Market Analysis, Insights and Forecast – By Country
7. Europe Biosimilars Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 7.1. Key Findings / Summary
- 7.2. Market Analysis, Insights and Forecast – By Product
- 7.2.1. Recombinant Glycosylated Proteins
- 7.2.1.1. Monoclonal Antibodies
- 7.2.1.2. Erythropoietin
- 7.2.1.3. Others
- 7.2.2. Recombinant Non-glycosylated Proteins
- 7.2.2.1. Insulin
- 7.2.2.2. Granulocyte Colony Stimulating Factor
- 7.2.2.3. Recombinant Human Growth Factor
- 7.2.2.4. Interferons
- 7.2.3. Recombinant Peptides
- 7.3. Market Analysis, Insights and Forecast – By Indication
- 7.3.1. Chronic Diseases
- 7.3.2. Oncology
- 7.3.3. Autoimmune Diseases
- 7.3.4. Infectious Diseases
- 7.3.5. Blood Disorders
- 7.3.6. Growth Hormone Deficiency
- 7.3.7. Others
- 7.4. Market Analysis, Insights and Forecast – By Country
- 7.4.1. UK
- 7.4.2. Germany
- 7.4.3. France
- 7.4.4. Italy
- 7.4.5. Spain
- 7.4.6. Russia
- 7.4.7. Rest of Europe
8. Asia Pacific Biosimilars Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 8.1. Key Findings / Summary
- 8.2. Market Analysis, Insights and Forecast – By Product
- 8.2.1. Recombinant Glycosylated Proteins
- 8.2.1.1. Monoclonal Antibodies
- 8.2.1.2. Erythropoietin
- 8.2.1.3. Others
- 8.2.2. Recombinant Non-glycosylated Proteins
- 8.2.2.1. Insulin
- 8.2.2.2. Granulocyte Colony Stimulating Factor
- 8.2.2.3. Recombinant Human Growth Factor
- 8.2.2.4. Interferons
- 8.2.3. Recombinant Peptides
- 8.3. Market Analysis, Insights and Forecast – By Indication
- 8.3.1. Chronic Diseases
- 8.3.2. Oncology
- 8.3.3. Autoimmune Diseases
- 8.3.4. Infectious Diseases
- 8.3.5. Blood Disorders
- 8.3.6. Growth Hormone Deficiency
- 8.3.7. Others
- 8.4. Market Analysis, Insights and Forecast – By Country
- 8.4.1. China
- 8.4.2. India
- 8.4.3. Japan
- 8.4.4. Australia
- 8.4.5. South East Asia
- 8.4.6. Rest of Asia Pacific
9. Latin America, Middle East and Africa Biosimilars Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 9.1. Key Findings / Summary
- 9.2. Market Analysis, Insights and Forecast – By Product
- 9.2.1. Recombinant Glycosylated Proteins
- 9.2.1.1. Monoclonal Antibodies
- 9.2.1.2. Erythropoietin
- 9.2.1.3. Others
- 9.2.2. Recombinant Non-glycosylated Proteins
- 9.2.2.1. Insulin
- 9.2.2.2. Granulocyte Colony Stimulating Factor
- 9.2.2.3. Recombinant Human Growth Factor
- 9.2.2.4. Interferons
- 9.2.3. Recombinant Peptides
- 9.3. Market Analysis, Insights and Forecast – By Indication
- 9.3.1. Chronic Diseases
- 9.3.2. Oncology
- 9.3.3. Autoimmune Diseases
- 9.3.4. Infectious Diseases
- 9.3.5. Blood Disorders
- 9.3.6. Growth Hormone Deficiency
- 9.3.7. Others
- 9.4. Market Analysis, Insights and Forecast – By Country
- 9.4.1. Brazil
- 9.4.2. Saudi Arabia
- 9.4.3. UAE
- 9.4.4. Rest of LAMEA
10. Competitive Analysis
- 10.1. Company Market Share Analysis, 2018
- 10.2. Key Industry Developments
- 10.3. Company Profile
- 10.4. Pfizer, Inc.
- 10.4.1. Business Overview
- 10.4.2. Segment 1 & Service Offering
- 10.4.3. Overall Revenue
- 10.4.4. Geographic Presence
- 10.4.5. Recent Development
*Similar details will be provided for the following companies
- 10.5. Intas Pharmaceuticals Ltd.
- 10.6. Biocon
- 10.7. Dr. Reddy’s Laboratories Ltd.
- 10.8. Teva Pharmaceutical Industries Ltd.
- 10.9. Sandoz International GmbH (A Novartis Division)
- 10.10. Celltrion Inc.
- 10.11. Amgen, Inc.
- 10.12. STADA Arzneimittel AG
Research Process
Data Library Research are conducted by industry experts who offer insight on
industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager
and SOFT) of the companies that active & prominent as well as the midsized organization
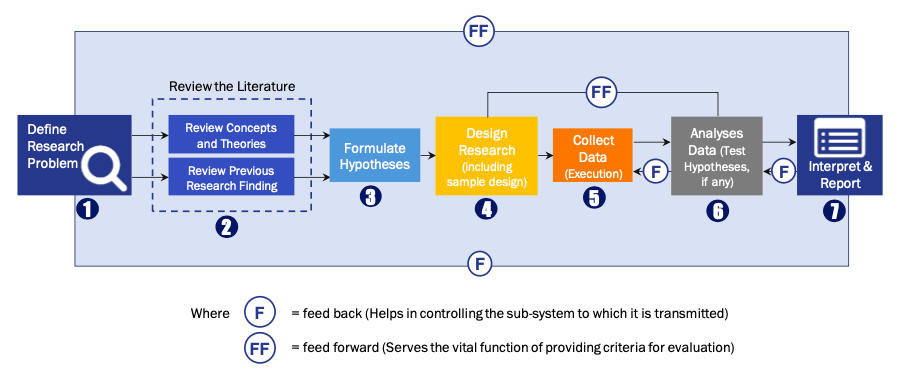
FIGURE 1: DLR RESEARH PROCESS
Primary Research
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary Research
Secondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size Estimation
Both, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model