Healthcare Contract Research Organization Market Overview The global healthcare contract research organization (CRO) market is expected to rise by increased funding for research and development programs. Several key advancements in the field of disease study and drug development have played an important role in the market’s growth. As well, in the current time, it is observed that tremendous growth in the medical and pharmaceutical research institutes. This is owing to the pressing need to develop new lines of treatment for chronic diseases and disorders. This key fact remarkably contributes to the growth of the global healthcare contract research organization (CRO) market in the coming years.
Furthermore, the investment for the global healthcare contract research organization (CRO) market has improved in recent times. Governments and public-sector organizations have made large investments towards improved medical research. This prime fact further boosts the market’s development in the forecast period.
Healthcare Contract Research Organization Market Segment OverviewBased on the type, the clinical trial services segment dominated the market in 2020 and accounted for the largest revenue share in the market. This is because it comprises four elaborate phases, including human subjects. Additionally, important factors such as the increasing need for innovative services within healthcare and the presence of a seamless industry for clinical trial and testing have played a curial role in the growth of the global healthcare contract research organization (CRO) market. Additionally, it is important to ensure the safety of research during drug development. This key fact will contribute to the market’s expansion in the forthcoming years.
Healthcare Contract Research Organization Market, By Type
- Drug Discovery
- Target Validation
- Lead Identification
- Lead Optimization
- Pre-clinical
- Clinical
- Phase I Trial Services
- Phase II Trial Services
- Phase III Trial Services
- Phase IV Trial Services
Healthcare Contract Research Organization Market, By Service
· Project Management/Clinical Supply Management
· Data Management
· Regulatory/Medical Affairs
· Medical Writing
· Clinical Monitoring
· Quality Management/ Assurance
· Bio-statistics
· Investigator Payments
· Laboratory
· Patient and Site Recruitment
· Technology
· Others
Healthcare Contract Research Organization Market Regional OverviewIn terms of region, North America held a significant revenue share in 2020. This can be attributed to the presence of several global players, who invest a major part of their revenue in research activities. Likewise, the market for healthcare contract research organizations (CRO) in the Asia Pacific has been expanding owing to the increasing outsourcing of medical services to India and China.
Healthcare Contract Research Organization Market, By Geography
· North America (US & Canada)
· Europe (UK, Germany, France, Italy, Spain, Russia & Rest of Europe)
· Asia-Pacific (Japan, China, India, Australia, & South Korea, & Rest of Asia-Pacific)
· LAMEA (Brazil, Saudi Arabia, UAE & Rest of LAMEA)
Healthcare Contract Research Organization Market Competitor overviewSome key developments and strategies adopted by manufacturers in Healthcare Contract Research Organization are highlighted below.
· In July 2021, Think Research Corporation, a company focused on transforming healthcare through digital health software solutions, announced that it has entered into a definitive share purchase agreement to acquire all of the issued and outstanding shares of Bio Pharma Services Inc., a leading contract research organization ("CRO") to pharmaceutical companies globally. This transaction is expected to nearly double the Company's pro forma 2021 revenue and presents significant new technology-driven revenue synergies for the combined entity.
Healthcare Contract Research Organization Market, Key Players· IQVIA Holdings Inc.
· Covance Inc.
· Pharmaceutical Product Development, LLC
· Parexel International Corporation
· Charles River Laboratories International, Inc.
· ICON, plc
· Medidata Solutions, Inc.
· Syneos Health
· Pharmaron
· GVK Biosciences Private Limited
· Wuxi AppTec
· MEDPACE HOLDINGS, INC.
· PRA Health Sciences
· CTI Clinical Trial & Consulting
Healthcare Contract Research Organization Market Study Global Market Analysis, Insights and Forecast, 2020-2027
1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
2. Executive Summary
3. Market Dynamics
- 3.1. Market Drivers
- 3.2. Market Restraints
- 3.3. Market Opportunities
4. Key Insights
- 4.1. Key Emerging Trends – For Major Countries
- 4.2. Latest Technological Advancement
- 4.3. Regulatory Landscape
- 4.4. Industry SWOT Analysis
- 4.5. Porters Five Forces Analysis
5. Global Healthcare Contract Research Organization Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 5.1. Key Findings / Summary
- 5.2. Market Analysis, Insights and Forecast – By Type
- 5.2.1. Drug Discovery
- 5.2.1.1. Target Validation
- 5.2.1.2. Lead Identification
- 5.2.1.3. Lead Optimization
- 5.2.2. Pre-clinical
- 5.2.3. Clinical
- 5.2.3.1. Phase I Trial Services
- 5.2.3.2. Phase II Trial Services
- 5.2.3.3. Phase III Trial Services
- 5.2.3.4. Phase IV Trial Services
5.3. Market Analysis, Insights and Forecast – By Service- 5.3.1. Project Management/Clinical Supply Management
- 5.3.2. Data Management
- 5.3.3. Regulatory/Medical Affairs
- 5.3.4. Medical Writing
- 5.3.5. Clinical Monitoring
- 5.3.6. Quality Management/ Assurance
- 5.3.7. Bio-statistics
- 5.3.8. Investigator Payments
- 5.3.9. Laboratory
- 5.3.10. Patient and Site Recruitment
- 5.3.11. Technology
- 5.3.12. Others
5.4. Market Analysis, Insights and Forecast – By Region- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Latin America, Middle East and Africa
6. North America Healthcare Contract Research Organization Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 6.1. Key Findings / Summary
- 6.2. Market Analysis, Insights and Forecast – By Type
- 6.2.1. Drug Discovery
- 6.2.1.1. Target Validation
- 6.2.1.2. Lead Identification
- 6.2.1.3. Lead Optimization
- 6.2.2. Pre-clinical
- 6.2.3. Clinical
- 6.2.3.1. Phase I Trial Services
- 6.2.3.2. Phase II Trial Services
- 6.2.3.3. Phase III Trial Services
- 6.2.3.4. Phase IV Trial Services
6.3. Market Analysis, Insights and Forecast – By Service- 6.3.1. Project Management/Clinical Supply Management
- 6.3.2. Data Management
- 6.3.3. Regulatory/Medical Affairs
- 6.3.4. Medical Writing
- 6.3.5. Clinical Monitoring
- 6.3.6. Quality Management/ Assurance
- 6.3.7. Bio-statistics
- 6.3.8. Investigator Payments
- 6.3.9. Laboratory
- 6.3.10. Patient and Site Recruitment
- 6.3.11. Technology
- 6.3.12. Others
6.4. Market Analysis, Insights and Forecast – By Country7. Europe Healthcare Contract Research Organization Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 7.1. Key Findings / Summary
- 7.2. Market Analysis, Insights and Forecast – By Type
- 7.2.1. Drug Discovery
- 7.2.1.1. Target Validation
- 7.2.1.2. Lead Identification
- 7.2.1.3. Lead Optimization
- 7.2.2. Pre-clinical
- 7.2.3. Clinical
- 7.2.3.1. Phase I Trial Services
- 7.2.3.2. Phase II Trial Services
- 7.2.3.3. Phase III Trial Services
- 7.2.3.4. Phase IV Trial Services
7.3. Market Analysis, Insights and Forecast – By Service- 7.3.1. Project Management/Clinical Supply Management
- 7.3.2. Data Management
- 7.3.3. Regulatory/Medical Affairs
- 7.3.4. Medical Writing
- 7.3.5. Clinical Monitoring
- 7.3.6. Quality Management/ Assurance
- 7.3.7. Bio-statistics
- 7.3.8. Investigator Payments
- 7.3.9. Laboratory
- 7.3.10. Patient and Site Recruitment
- 7.3.11. Technology
- 7.3.12. Others
7.4. Market Analysis, Insights and Forecast – By Country- 7.4.1. UK
- 7.4.2. Germany
- 7.4.3. France
- 7.4.4. Italy
- 7.4.5. Spain
- 7.4.6. Russia
- 7.4.7. Rest of Europe
8. Asia Pacific Healthcare Contract Research Organization Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 8.1. Key Findings / Summary
- 8.2. Market Analysis, Insights and Forecast – By Type
- 8.2.1. Drug Discovery
- 8.2.1.1. Target Validation
- 8.2.1.2. Lead Identification
- 8.2.1.3. Lead Optimization
- 8.2.2. Pre-clinical
- 8.2.3. Clinical
- 8.2.3.1. Phase I Trial Services
- 8.2.3.2. Phase II Trial Services
- 8.2.3.3. Phase III Trial Services
- 8.2.3.4. Phase IV Trial Services
8.3. Market Analysis, Insights and Forecast – By Service- 8.3.1. Project Management/Clinical Supply Management
- 8.3.2. Data Management
- 8.3.3. Regulatory/Medical Affairs
- 8.3.4. Medical Writing
- 8.3.5. Clinical Monitoring
- 8.3.6. Quality Management/ Assurance
- 8.3.7. Bio-statistics
- 8.3.8. Investigator Payments
- 8.3.9. Laboratory
- 8.3.10. Patient and Site Recruitment
- 8.3.11. Technology
- 8.3.12. Others
8.4. Market Analysis, Insights and Forecast – By Country- 8.4.1. China
- 8.4.2. India
- 8.4.3. Japan
- 8.4.4. Australia
- 8.4.5. South East Asia
- 8.4.6. Rest of Asia Pacific
9. Latin America, Middle East and Africa Healthcare Contract Research Organization Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 9.1. Key Findings / Summary
- 9.2. Market Analysis, Insights and Forecast – By Type
- 9.2.1. Drug Discovery
- 9.2.1.1. Target Validation
- 9.2.1.2. Lead Identification
- 9.2.1.3. Lead Optimization
- 9.2.2. Pre-clinical
- 9.2.3. Clinical
- 9.2.3.1. Phase I Trial Services
- 9.2.3.2. Phase II Trial Services
- 9.2.3.3. Phase III Trial Services
- 9.2.3.4. Phase IV Trial Services
9.3. Market Analysis, Insights and Forecast – By Service- 9.3.1. Project Management/Clinical Supply Management
- 9.3.2. Data Management
- 9.3.3. Regulatory/Medical Affairs
- 9.3.4. Medical Writing
- 9.3.5. Clinical Monitoring
- 9.3.6. Quality Management/ Assurance
- 9.3.7. Bio-statistics
- 9.3.8. Investigator Payments
- 9.3.9. Laboratory
- 9.3.10. Patient and Site Recruitment
- 9.3.11. Technology
- 9.3.12. Others
9.4. Market Analysis, Insights and Forecast – By Country- 9.4.1. Brazil
- 9.4.2. Saudi Arabia
- 9.4.3. UAE
- 9.4.4. Rest of LAMEA
10. Competitive Analysis
- 10.1. Company Market Share Analysis, 2018
- 10.2. Key Industry Developments
- 10.3. Company Profile
- 10.4. IQVIA Holdings Inc.
- 10.4.1. Business Overview
- 10.4.2. Segment 1 & Service Offering
- 10.4.3. Overall Revenue
- 10.4.4. Geographic Presence
- 10.4.5. Recent Development
*Similar details will be provided for the following companies
- 10.5. Covance Inc.
- 10.6. Pharmaceutical Product Development, LLC
- 10.7. Parexel International Corporation
- 10.8. Charles River Laboratories International, Inc.
- 10.9. ICON, plc
- 10.10. Medidata Solutions, Inc.
- 10.11. Syneos Health
- 10.12. Pharmaron
- 10.13. GVK Biosciences Private Limited
- 10.14. Wuxi AppTec
- 10.15. MEDPACE HOLDINGS, INC.
- 10.16. PRA Health Sciences
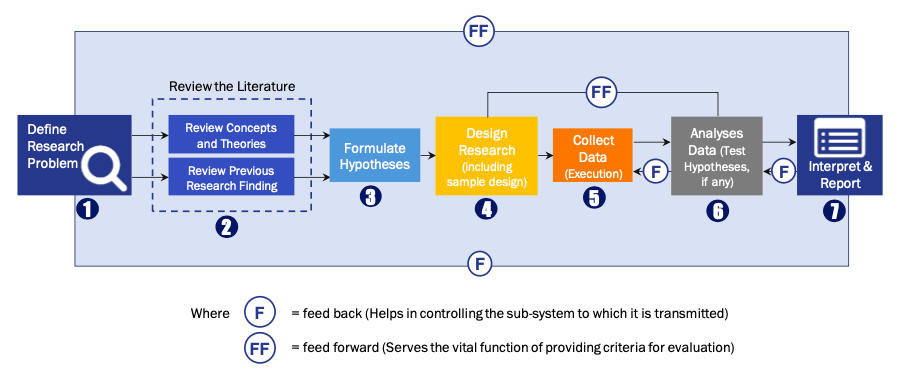
Research Process
Data Library Research are conducted by industry experts who offer insight on
industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager
and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
Primary Research
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary Research
Secondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size Estimation
Both, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model