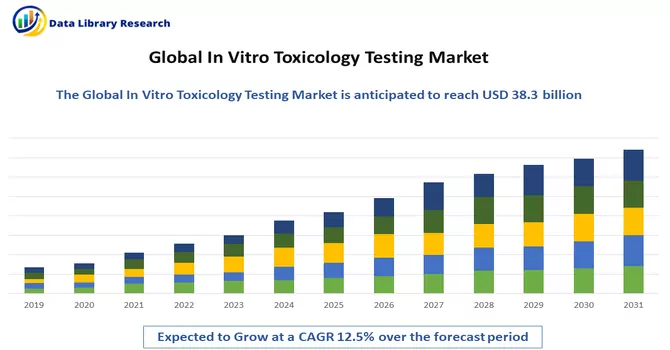
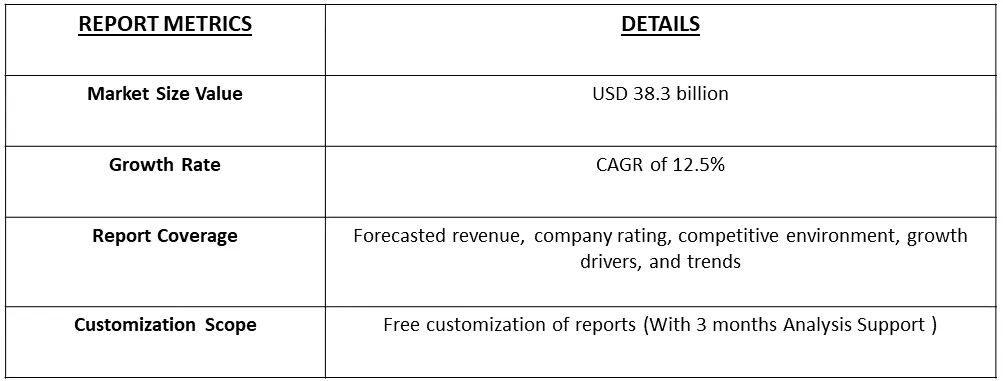
The global in-vitro toxicology testing market size was estimated at USD 38.3 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 12.5% from 2024 to 2031.
Get Complete Analysis Of The Report - Download Free Sample PDF
The in vitro toxicology testing market refers to the industry involved in conducting assessments of potential toxic effects of substances using methods that do not involve live organisms. "In vitro" literally means "in glass" and in this context, it signifies testing conducted in a controlled laboratory environment outside a living organism. In vitro toxicology testing plays a crucial role in the pharmaceutical, cosmetics, chemicals, and other industries to evaluate the safety of new compounds and products before they are introduced to humans or the environment. This testing utilizes cell cultures, tissues, or isolated cellular components to assess the potential toxicity of substances. It provides insights into mechanisms of toxicity, identifies potential hazards, and helps in the risk assessment of chemicals and pharmaceuticals. In vitro toxicology methods have become essential in regulatory submissions, allowing for more humane and efficient screening of substances compared to traditional in vivo (animal) testing. Key aspects of in vitro toxicology testing include assessing cytotoxicity, genotoxicity, carcinogenicity, and other adverse effects on cellular systems. The market for in vitro toxicology testing continues to grow as advancements in cell culture technologies, high-throughput screening, and molecular biology techniques enhance the accuracy and efficiency of these tests, meeting regulatory requirements and contributing to the development of safer products in various industries.
The expansion of the market can be credited to the ongoing progress in toxicology research and the continuous advancement of technologies. Furthermore, the increasing focus on the development of personalized medicines is anticipated to provide a significant impetus to the market in the forecast period. There is a growing awareness regarding the limitations and challenges associated with traditional methods of toxicology screening. This awareness has resulted in a more widespread acceptance of in-vitro testing as a valuable and reliable tool for assessing the safety and effectiveness of compounds. As a result, the market is experiencing a positive trajectory, driven by the recognition of the advantages offered by in-vitro testing in overcoming the shortcomings of conventional toxicology screening methods.
The In Vitro Toxicology Market is witnessing notable trends driven by continuous technological advancements, including microscale systems and 3D cell culture models, enhancing the precision and relevance of toxicity testing. The growing demand for personalized medicine is a significant driver, requiring thorough in vitro toxicity profiling for tailored treatments. Regulatory acceptance is increasing, favoring in vitro methods, and awareness of animal welfare concerns is steering the industry towards cruelty-free testing alternatives. The integration of artificial intelligence and computational models, collaborations across academia and industry, and a focus on environmental toxicology further characterize the evolving landscape, illustrating a dynamic market responding to advancements and ethical considerations in toxicity assessment.
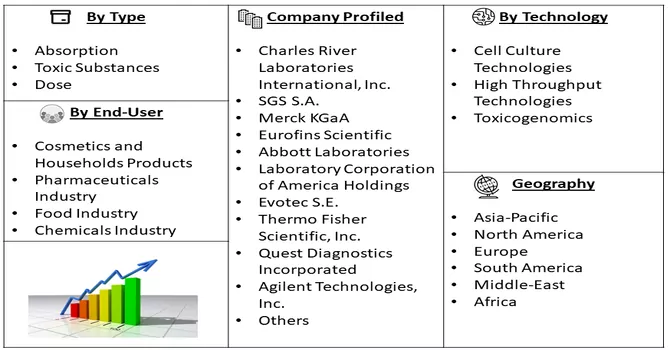
Market Segmentation: The In-vitro Toxicology Testing Market is Segmented by Type (Absorption, Toxic Substances, Dose), by Technology (Cell Culture Technologies, High Throughput Technologies, Toxicogenomics), by End User (Cosmetics and Households Products, Pharmaceuticals Industry, Food Industry, Chemicals Industry) and by geography (North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa). The report offers market size and forecasts for the global credit card market in value (USD billion) for all the above segments.
For Detailed Market Segmentation - Download Free Sample PDF
Market Drivers:
Increasing focus on ethical considerations and the global movement towards reducing animal testing has driven the adoption of in vitro methods.
The increasing emphasis on ethical considerations and the global movement towards reducing animal testing have significantly influenced the adoption of in vitro methods in toxicology research. Ethical concerns surrounding the use of animals in testing procedures, coupled with a growing awareness of animal welfare, have prompted a shift towards more humane and alternative approaches. In vitro methods, which involve testing on cells or tissues outside of a living organism, have emerged as a ethical and scientifically advanced solution to traditional animal testing practices. The ethical considerations stem from the recognition that animals used in toxicity testing may experience pain and distress, raising moral and welfare concerns. This realization has led to a collective global effort to minimize or replace animal testing whenever possible. In vitro methods offer a more humane alternative, allowing researchers to conduct experiments in controlled laboratory environments without the need for live animals. The global movement towards reducing animal testing aligns with regulatory initiatives and guidelines that encourage the development and adoption of alternative methods. Various countries and regulatory bodies are actively promoting the use of in vitro toxicology as a reliable and effective means to assess the safety and potential risks of substances, including pharmaceuticals, chemicals, and consumer products. This shift reflects a broader commitment to advancing scientific practices that prioritize both research validity and ethical responsibility. The adoption of in vitro methods not only addresses ethical concerns but also provides practical benefits. In vitro testing allows for more accurate predictions of human responses to substances, as human-derived cells and tissues are used in these assays. This enhances the relevance and reliability of toxicity assessments, contributing to the overall improvement of research outcomes. Thu, the increasing focus on ethical considerations and the global movement to reduce animal testing underscore the pivotal role of in vitro methods in modern toxicology research. This shift reflects a conscientious effort by the scientific community and regulatory bodies to embrace innovative, humane, and ethically sound approaches to advance research while ensuring the welfare of animals.
Advancements in technology, such as 3D cell culture models and organ-on-a-chip platforms, enhance the accuracy and relevance of toxicity assessments, driving demand for innovative in vitro solutions
The landscape of toxicology research is undergoing a transformative phase propelled by significant advancements in technology. Innovations such as 3D cell culture models and organ-on-a-chip platforms are revolutionizing the accuracy and relevance of toxicity assessments, fostering a robust demand for cutting-edge in vitro solutions. The integration of 3D cell culture models represents a departure from traditional two-dimensional methods, providing a more physiologically relevant environment for cells. This advancement allows researchers to mimic complex tissue structures and cellular interactions, yielding toxicity data that better mirrors in vivo conditions. As a result, the predictive capabilities of these models enhance, providing a more accurate representation of how substances may behave within the human body. Similarly, organ-on-a-chip platforms simulate the microenvironment of specific organs, allowing researchers to study the effects of substances on organ-level functions. These microfluidic systems replicate key aspects of organ physiology, enabling a dynamic and real-time analysis of toxicity responses. This technology addresses the limitations of static in vitro models and contributes to a more comprehensive understanding of how drugs or chemicals may impact different organs. The demand for these innovative in vitro solutions is driven by the pharmaceutical, biotechnology, and chemical industries, as well as regulatory bodies seeking more predictive and reliable toxicity assessments. The ability to generate data that closely correlates with in vivo outcomes reduces the reliance on animal testing, aligning with ethical considerations and regulatory trends favoring alternative methods.
Moreover, the adoption of advanced technologies in toxicology research enhances the efficiency of drug development pipelines. Researchers can conduct high-throughput screening using 3D models and organ-on-a-chip platforms, expediting the identification of potential toxicities and enabling data-driven decision-making in the early stages of drug discovery. Thus, the advancements in technology, particularly 3D cell culture models and organ-on-a-chip platforms, signify a paradigm shift in toxicity assessments. These innovations not only enhance accuracy and relevance but also contribute to the ongoing efforts to refine in vitro methodologies, paving the way for more effective and ethical toxicology research practices.
Market Restraints:
Despite the positive trajectory, the In Vitro Toxicology Market faces certain restraints. One significant challenge lies in replicating the complex in vivo environment accurately. Achieving a holistic representation of the human body's response to various substances remains a hurdle, impacting the predictive capabilities of in vitro models. Standardization and validation of in vitro assays present another challenge, as ensuring consistency and reliability across different laboratories is crucial for regulatory acceptance. Additionally, the high upfront costs associated with implementing advanced in vitro technologies may limit their widespread adoption, especially among smaller research institutions. The need for continuous technological advancements to keep pace with evolving toxicity testing requirements poses an ongoing challenge. Addressing these constraints requires collaborative efforts from industry stakeholders, researchers, and regulatory bodies to ensure the continued growth and effectiveness of in vitro toxicology methodologies.
The In Vitro Toxicology Market underwent significant shifts during the COVID-19 pandemic, presenting a blend of challenges and opportunities. Immediate disruptions ensued with lockdowns and closures impacting laboratory activities, delaying in vitro toxicology research. Resources and attention were redirected towards COVID-19 research, affecting the allocation of resources in other areas, including in vitro toxicology. The heightened focus on public health increased emphasis on drug safety, potentially benefiting the in vitro toxicology market. Digital technologies and remote work gained prominence, enhancing communication and collaboration within the industry. Supply chain disruptions posed challenges, impacting the workflow of research activities. The pandemic underscored ethical considerations, potentially driving increased demand for in vitro alternatives to traditional testing methods. Additionally, in vitro toxicology methods found applications in COVID-19 research, showcasing versatility in addressing emerging health challenges. The long-term impact hinges on the industry's adaptability to evolving landscapes and its ability to address broader implications on research and public health priorities.
Segmental Analysis:
Toxic Substances Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
Toxic substances play a pivotal role in shaping the dynamics of the in-vitro toxicology market, as industries across pharmaceuticals, chemicals, cosmetics, and environmental sciences increasingly rely on advanced testing methodologies to assess the safety and potential risks associated with these substances. The in-vitro toxicology market is instrumental in providing comprehensive evaluations of toxic effects without the need for traditional animal testing, aligning with ethical considerations and regulatory demands. As the global awareness of the adverse impacts of toxic substances continues to grow, the demand for in-vitro toxicology solutions is on the rise. The market responds to the need for accurate, cost-effective, and high-throughput testing methods to assess the toxicity of various substances, including pharmaceutical drugs, chemicals, and consumer products. In-vitro models facilitate the screening of potential toxic effects, offering insights into mechanisms of action, dose-response relationships, and other crucial parameters. The market's focus on toxic substances extends to addressing challenges associated with genotoxicity, carcinogenicity, and other adverse effects. In-vitro toxicology methods, including cell-based assays, 3D cell culture models, and advanced technologies such as organ-on-a-chip, provide a more comprehensive understanding of the potential harm posed by toxic substances. The regulatory landscape also plays a vital role in driving the in-vitro toxicology market's response to toxic substances. Stringent regulatory requirements and a shift toward alternative testing methods contribute to the market's growth, as organizations seek efficient and reliable ways to comply with safety standards. Thus, the in-vitro toxicology market serves as a critical player in the assessment and mitigation of risks associated with toxic substances. With a focus on advancing technologies, ethical considerations, and regulatory compliance, the market continues to evolve to meet the complex challenges posed by toxicological evaluations in various industries.
High Throughput Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
The High Throughput Segment in the In Vitro Toxicology Market represents a crucial facet characterized by advanced technologies and methodologies designed to streamline and expedite toxicity screening processes. This segment plays a pivotal role in meeting the escalating demand for rapid and efficient testing solutions in various industries, including pharmaceuticals, chemicals, and cosmetics. High throughput in vitro toxicology involves the simultaneous assessment of multiple samples, allowing for the screening of a large number of compounds or substances in a shorter time frame. One of the key drivers of the High Throughput Segment is the need for comprehensive toxicity assessments during the early stages of drug discovery and development. As the pharmaceutical and biotechnology industries continue to expand, there is an increasing demand for innovative and rapid testing methodologies to evaluate the safety profiles of potential drug candidates. High throughput in vitro toxicology addresses this demand by enabling the simultaneous analysis of numerous compounds, facilitating data-driven decision-making in drug development pipelines. Advancements in automation, robotics, and data analysis have significantly contributed to the efficiency of high throughput screening methods. Automated systems allow for the parallel processing of samples, minimizing manual intervention and reducing the time required for toxicity assessments. This results in enhanced productivity, cost-effectiveness, and the ability to handle large-scale testing initiatives. Moreover, the High Throughput Segment aligns with the broader industry trend of shifting away from traditional animal testing methods. As ethical considerations gain prominence and regulatory bodies encourage alternative testing approaches, high throughput in vitro toxicology emerges as a vital solution. It provides a platform for conducting thorough toxicity screenings without relying on animal models, meeting both scientific and ethical imperatives. Thus, the High Throughput Segment in the In Vitro Toxicology Market stands as a cornerstone of innovation, offering a rapid and efficient means of toxicity screening for a wide range of applications. As technology continues to evolve and the demand for quicker and more reliable testing methods persists, this segment is poised to play a central role in shaping the future of in vitro toxicology.
Pharma and Biotech Companies Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
Pharmaceutical and biotechnology companies play a pivotal role in driving the growth and evolution of the In Vitro Toxicology Market. As leaders in drug discovery, development, and manufacturing, these companies heavily rely on advanced toxicity testing methodologies to ensure the safety and efficacy of their products. The in vitro toxicology market caters to the diverse needs of pharmaceutical and biotech companies by providing innovative and efficient testing solutions, aligning with ethical considerations, and meeting stringent regulatory requirements. In drug development, the early identification of potential toxicities is critical to the success of pharmaceutical and biotech projects. In vitro toxicology methods offer a cost-effective and time-efficient approach to screening compounds for safety, allowing companies to make informed decisions about which candidates to advance in their pipelines. This is particularly important in the context of regulatory expectations for comprehensive safety assessments. Pharmaceutical and biotech companies also benefit from the versatility of in vitro toxicology in addressing various aspects of drug development, including genotoxicity, carcinogenicity, and organ-specific toxicities. The ability to assess these parameters using advanced cell culture models and high-throughput screening methods contributes to a more thorough understanding of potential risks associated with drug candidates. Additionally, the commitment to ethical considerations has driven pharmaceutical and biotech companies to actively seek alternatives to traditional animal testing methods. In vitro toxicology offers a humane and scientifically advanced approach, aligning with the industry's growing emphasis on social responsibility and ethical research practices. Collaborations between in vitro toxicology solution providers and pharmaceutical and biotech companies further enhance the development and adoption of cutting-edge testing technologies. Joint efforts often lead to the creation of tailored solutions that address specific challenges in drug development, fostering a synergistic relationship between these entities. Thus, the partnership between pharmaceutical and biotech companies and the In Vitro Toxicology Market is instrumental in advancing drug discovery, ensuring product safety, and navigating the complexities of regulatory compliance.
North America Region is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
North America holds a dominant position in the In Vitro Toxicology Market, reflecting a robust landscape driven by factors such as technological advancements, a strong emphasis on research and development, and a well-established pharmaceutical and biotechnology industry. As of 2023, North America accounted for a significant share of the market, underlining its pivotal role in shaping the trajectory of in vitro toxicology practices. One key contributing factor to North America's prominence in the in vitro toxicology sector is the high incidence of respiratory disorders and the region's substantial investments in biotechnology. Leading pharmaceutical and biotech companies in North America are at the forefront of adopting innovative technologies for drug research and development, creating a substantial demand for advanced toxicity testing methods. Government organizations such as the FDA and the U.S. Department of Health and Human Services, in collaboration with institutions like the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), play a crucial role in setting and enforcing stringent regulatory guidelines. This regulatory framework encourages the adoption of in vitro testing methodologies, contributing to the market's growth. The region's response to the COVID-19 pandemic further highlighted North America's resilience and adaptability. While the initial disruptions affected research activities, the focus on combatting the pandemic led to an increased emphasis on drug safety and efficacy, potentially benefiting the in vitro toxicology market. The acceleration of digital technologies and remote work practices during the pandemic also influenced the communication and collaboration landscape within the industry. The North American in vitro toxicology market encompasses a wide range of innovative companies and research institutions actively contributing to advancements in toxicity testing. The region's commitment to ethical considerations and the adoption of alternatives to traditional animal testing aligns with global trends, further fostering growth in the in vitro toxicology sector. Thus, North America's leadership in the In Vitro Toxicology Market is characterized by its technological prowess, robust regulatory framework, and the collaborative efforts of key industry players and regulatory bodies. As the region continues to be a hub for pharmaceutical and biotechnology advancements, its influence on the in vitro toxicology landscape is expected to remain significant in the coming years.
Get Complete Analysis Of The Report - Download Free Sample PDF
Up-and-coming entrants in the in-vitro toxicology testing market, such as InSphero and MatTek Corporation, are actively capitalizing on cutting-edge technologies and forging strategic alliances to establish a strong presence in the industry. These companies are at the forefront of innovation, with initiatives encompassing the development of sophisticated 3D cell culture models and advanced organ-on-a-chip platforms. Their strategic focus is on disrupting the market landscape by introducing novel and highly predictive in-vitro toxicology solutions. This strategic approach is in direct response to the escalating demand for alternatives to traditional animal testing methods, aligning with the global shift towards more ethical and scientifically advanced approaches in toxicity assessment. As these emerging players continue to pioneer advancements, their contributions are poised to shape the future of in-vitro toxicology testing, offering more reliable and humane testing methodologies. Key In-vitro Toxicology Testing Companies:
Recent Development:
1) In April 2023, Thermo Fisher Scientific marked a significant milestone by introducing its inaugural real-time PCR assay kit, 37 CE-IVD, designed specifically for the testing of infectious diseases. This launch represents a noteworthy advancement in Thermo Fisher Scientific's portfolio, contributing to the company's commitment to providing cutting-edge solutions in the field of molecular diagnostics and infectious disease testing.
2) In April 2023, Gentronix, Ltd demonstrated its dedication to meeting the increasing demand within the industry by expanding its laboratory facilities. This expansion initiative signifies Gentronix's proactive response to the growing needs of the market. By bolstering its laboratory capacity, Gentronix aims to enhance its capabilities in delivering high-quality services, accommodating a surge in demand for its offerings, and positioning itself as a key player in the evolving landscape of laboratory services and testing. Overall, these developments highlight the dynamic and innovative nature of companies within the life sciences sector as they strive to address emerging challenges and capitalize on opportunities in the rapidly evolving field of diagnostic testing and research.
Q1. What is the current In Vitro Toxicology Testing Market size?
The global in-vitro toxicology testing market size was estimated at USD 38.3 billion in 2023.
Q2. What is the market size of the In Vitro Toxicology Testing Market?
In Vitro Toxicology Testing Market is expected to grow at a compound annual growth rate (CAGR) of 12.5% over the forecast period.
Q3. What segments are covered in the In Vitro Toxicology Testing Market Report?
By Type, By Application, End User & Geography these are the segments covered in the In Vitro Toxicology Testing Market Report.
Q4. Which region has the largest share of the In Vitro Toxicology Testing Market? What are the largest region's market size and growth rate?
North America has the largest share of the market . For detailed insights on the largest region's market size and growth rate request a sample here
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS

Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model
