The clinical trial supply market is currently valued at USD 4.78 billion in the year 2022 and is expected to register a CAGR of 8.5% over the forecast period, 2022-2031.
The clinical trials supply market includes various tools that contribute to conducting scientific research experiments, investigations, and other clinical trial experiments. The commonly used products in the clinical trials supply market are infusion pumps, nebulizers, and needles. These are used for conducting trials which are used for detecting, conducting, and treatment of disease. Also, these have a high contribution to carrying out respiratory, cardiological, and oncological procedures.
Clinical trial management has a vital role in the pharmaceutical R&D sector. CROs (Clinical trial Organizations) are responsible for carrying out clinical trials of new drugs. This clinical trial supply market is globalized due to its better supply. Also, the coming of various biopharmaceutical companies into the market led to the growth of the clinical trial supply market.
Diseases such as HIV and cancer drive the growth of this market. In this present scenario, (Contract Research Organization) CROs are also driving the market growth. CROs approach pharmaceutical and biotechnological companies thus increasing the supply of clinical trial supply market. Also, the R&D sector is developing and now also funded by governmental and non-governmental sectors which is also another reason for the increase in supply.
Several technology trends that are set to further revolutionize the design, conduct, and analysis of trials include decentralized trials, wearable devices, machine learning, and Risk-Based Quality Management (RBQM). AI and Machine learning are becoming increasingly important in clinical trials, as they provide a way to analyze large amounts of data and make predictions about outcomes. From identifying potential trial participants to predicting patient responses to treatment, Machine Learning can help make clinical trials more efficient and effective.
Additionally, these technologies can help automate routine tasks, freeing up time and resources that can be used to focus on other aspects of the trial. Some of the AI & and ML applications in clinical trials include.
Applications :
Segmentation :
The clinical trial supply market is segmented
By Clinical Phase outlook
Clinical Trials Product Outlook
Clinical Trial Therapeutic-use Outlook
Geography :
The report offers the value (in USD million) for the above segments.
Drivers:
Increasing R&D Expenditure of Pharmaceutical and Biopharmaceutical Companies
The spending on the R&D market and the introduction of new drugs have increased in the past two decades. For instance, an article published in Congressional Budget Office, in 2021 reports that in the year 2019, the pharmaceutical industry spent 83 billion dollars on R&D, this amount was 10 times what the industry used to spend in the 1980s. Additionally, the number of new drug approvals increased up to 60% between 2010 to 2020, with a peak of 50 novel approvals of drugs by the Food and Drug Administration (FDA) in 2021.
As per the data reported on the clinicaltrials.gov site, in June 2022, the total number of clinical trial studies globally reported was 419,487 while 2,119 were registered clinical trials in 2000. The increase in expenditure in the R&D market has boosted the novel drug development process, which has boosted the number of clinical trials, thereby driving the clinical trials supply market.
Restraints:
High Cost of The Drug Development Market and Research
The data published by the London School of Economics in May 2020, reported that the researchers behind this new study estimated that the median cost of bringing a new drug to market was USD 985 million, and the average cost was USD 1.3 billion. Thus, such a high cost of drug development may slow down the growth of the studied market.
The COVID-19 pandemic has had a substantial impact on the clinical trial supply market initially. The strict lockdowns and government regulations intended to slow down the spread of COVID-19 resulted in a decrease in demand for products across aesthetic segments. Thus, the COVID-19 outbreak affected the market's growth adversely in its preliminary phase; however, the market is expected to gain traction due to the significant increase in clinical trial supply globally. In the current scenario, due to the decrease in COVID-19 cases and ease in restrictions, the market is expected to witness significant growth over the forecast period.
Segmental Analysis:
Phase 3 Clinical Trials are expected to Witness Significant growth over the Forecast Period
Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants. Phase 3 studies provide most of the safety data.
Supply Chain Management Segment is Expected to Witness Significant growth over the Forecast Period
Pharmaceutical supply chain management consists of the strategic coordination of the entire value-added process of a product (pharma value chain) and the logistics. Using an efficient, reliable supply chain directly impacts customer satisfaction, cost containment, and competitive differentiation. This is because customers want consistent quality drugs that work every time they are administered to patients. Thus, the segment is expected to witness significant growth over the forecast period.
Oncology segment is Expected to Witness Significant growth Over the Forecast Period
Cancer clinical trials are medical research trials involving people with cancer. The oncology therapeutics alone accounts for around 20% of the global sales of the pharmaceuticals. Therefore, the rising importance and higher potential of profit are attracting the market players to invest in the oncology market.
North America had the largest regional market for clinical trial supplies
North America had the largest market for clinical trial supply market due to the growing number of registered clinical trials due to the presence of a large number of CROs has driven the growth of the clinical trial supply market in North America.
Pharmaceutical companies such as Pfizer, Abbott Labs, and Johnson and Johnson are present in North America and responsible for the largest regional market supply in North America. For instance, in clinical trials logistic survey in 2021, states that about 60% of clinical trials in phase 2 are outsourced.
Due to the high concentration of pharmaceutical leaders, innovative combinations of approved and novel treatments have driven the number of phase 2 trial studies. The high prevalence of cancer in the United States led to high investment in R&D is another driving feature of the clinical trial supply market in North America. For instance, Cancer.Org Cancer Facts and Figure accounted for 609,360 new cancer cases in 2022 which was 0.12 % higher than reported in 2021.
Thus, an increasing incidence of cancer cases reported every year in North America is driving the clinical trial supply market in that region.
The Clinical Trial Supply market is fragmented in nature due to the presence of several companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international as well as local companies which hold market shares key players in the clinical trial supplies market include
Key Players :
Recent Developments
Q1. What is the Growth Rate of the Clinical Trial Supply Chain Market ?
Clinical Trial Supply Chain Market is expected to register a CAGR of 8.5% over the forecast period, 2022-2031.
Q2. Which Region is expected to hold the highest Market share ?
North America had the largest market for clinical trial supply market
Q3. What segments are covered in the Clinical Trial Supply Chain Market Report?
Clinical Trial Supply Chain Market Report covers segments like- By Clinical Phase outlook, Clinical Trials Product Outlook,Clinical Trial Therapeutic-use Outlook and Geography.
Q4. Which is the leading Component Segment in the Clinical Trial Supply Chain Market?
Oncology segment is Expected to Witness Significant growth Over the Forecast Period
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
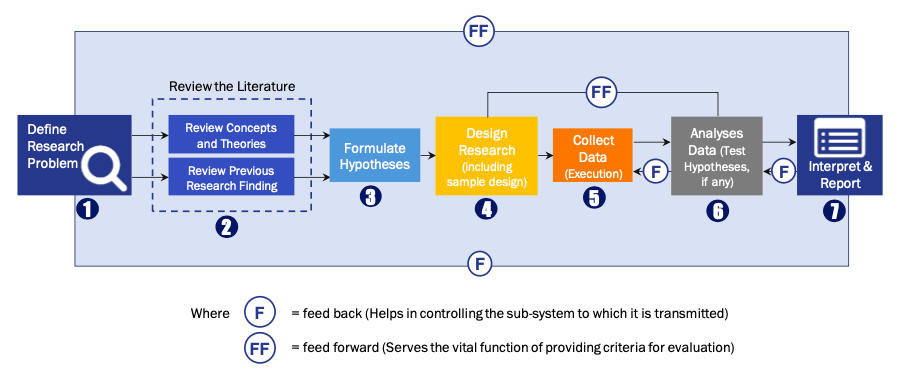
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model